Introduction:
In 1981 a few young homosexual men died in Los Angeles from a rare type of pneumonia. In June of 1981 the Centers for Disease Control and Prevention (CDC) published their first report on this rare type of pneumonia and called it Pneumocystis Carinii Pneumonia (PCP). By July of 1982 the number of cases of this rare disease had increased among homosexuals, intravenous drug users and hemophiliacs. The immune system of the patients with this disease seemed to collapse for no apparent reason, so the U.S. health officials called the disease acquired immune deficiency syndrome (AIDS).
During the end of 1982, the first case of AIDS resulting from blood transfusion was documented. This led the U.S. government to warn people that the blood supply might be contaminated. Researchers studied the pattern of the disease and found that it could be spread through sexual intercourse, contaminated needles, from infected mothers to their fetuses before birth, and by transfusion of bodily fluids from one person to another. In this article I focus on some of the research that has taken place in order to stop the spread of the virus, and the different types of drugs that have been successful in slowing down the virus. The mechanisms of these drugs will not be discussed in this article due to the immense information, which would require a separate article in order to give justice to the topic. Before I begin, I would like to give credit to the scientists who discovered the virus, explain the difference between HIV and AIDS, and briefly discuss the structure and life cycle of HIV.
The discovery of HIV virus:
This image was provided by Dr. John
W. Kimball
http://www.ultranet.com/~jkimball/BiologyPages/
In May 1983, Luc Montagnier, a virologist at the Pasteur Institute in Paris, and his team were the first to be successful in isolating a new human retrovirus from an AIDS patient. The results were published in Science and were ignored. Retroviruses are viruses that have a single-stranded RNA as their genetic material, as shown in the above diagram. Their RNA is copied by a viral enzyme called reverse transcriptase, and forms a double-stranded DNA molecule. Two strands of RNA coiled together as a double helix form DNA. The literal meaning of retrovirus is "backward viruses". This comes from the reversal flow of the genetic material, which is normally from DNA to RNA. In retroviruses the genetic material flows from RNA to DNA, and this process is called reverse transcription.
In April 1984, Robert Gallo of the National Cancer Institute (NCI) in Bethesda, Maryland announced that his laboratory had identified the retrovirus that causes AIDS. In January 1985, Montagnier and Gallo each published the genetic sequence of the viruses they had identified, and they both shared the credit for the isolation of the HIV virus. The name Human Immunodeficiency Virus (HIV) was given to the retrovirus by an international committee, which was held in 1986.
What is AIDS?
AIDS stands for Acquired Immune Deficiency Syndrome. In general people tend to use the acronyms AIDS and HIV interchangeably, but they each describe different conditions. All people who have AIDS have HIV, but not all people with HIV have AIDS. AIDS is caused by the HIV virus and is considered the terminal stage of HIV disease. During the course of infection the virus gradually deteriorates the cluster designation 4 (CD4) T cells of the immune system. These CD4 T cells play a critical role in the immune response, they signal other cells in the immune system to perform their special defensive functions against microbes and bacteria that are harmful to the human body.
There is a long incubation
period between infection with HIV and the onset of AIDS; this period averages
about 10 years. Since HIV damages the immune systemís ability to fight
off disease, the body is left susceptible to opportunistic infections.
A person is labeled as an AIDS patient when their blood concentration of
CD4 T cells is very low. A healthy person who is not infected by HIV has
a CD4 T cell count of 800-1,200 T cells per microliter of blood. The CDC
has classified the process of HIV infection into three clinical categories:
- Category A: The infection may be asymptomatic or cause persistent lymphadenopathy
(swollen lymph nodes). The blood concentration of CD4 T cells is 500 per
microliter or above.
Category B: Certain symptoms appear as the CD4 T cell counts fall into the 200-499 per microliter range and the immune system weakens. Especially common are persistent infections by the yeastlike fungus Candida albicans, which can appear in the mouth, throat, or vagina. Other conditions may include shingles, persistent diarrhea and fever, whitish patches on the oral mucosa (hairy leukoplakia), and certain cancerous or precancerous conditions such as cervical (of the cervix) growths.
Category C: This stage is clinical AIDS. The CD4 T cell count is 200 per microliter or lower. Less than 14% of the T cell present are CD4 T cells. Many AIDS indicator conditions now appear. Important among them are Candida albicans infections of the esophagus, bronchi, and lungs; cytomegalovirus eye infections; tuberculosis; Pneumocystis pneumonia; toxoplasmosis of the brain; and Kaposiís sarcoma.1
AIDS patients do not
have enough CD4 T cells to fight the various diseases often encountered,
therefore AIDS is not considered as a single disease but a combination
of different diseases and cancers. The symptoms often associated with these
diseases are commonly diarrhea, weight loss, infections of the lungs, eyes,
brain, and other organs. The cause of death in people with AIDS is the
underlying viral infections, which are due to the deterioration of immune
cells. "Pneumocystis Carinii pneumonia (PCP, caused by a protozoan parasite)
and Kaposiís sarcoma (KS, a formerly rare cancer) have been the most common
causes of death in people with AIDS in the U.S."2
What is HIV?
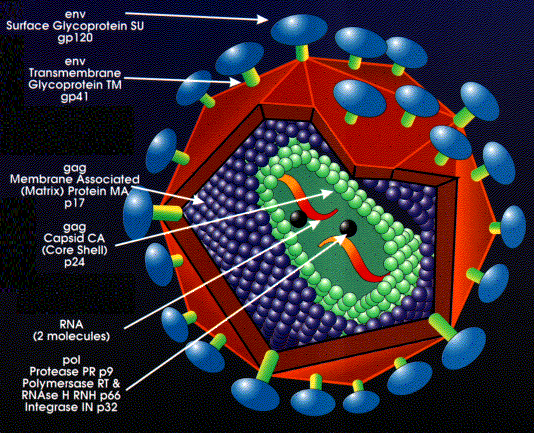
HIV stands for Human Immunodeficiency Virus, and it is a retrovirus that belongs to a subgroup known as lentiviruses, which causes AIDS. "Retroviruses are RNA viruses whose life cycle involves a DNA intermediate that is integrated into the host genome."3 Lentiviruses are characterized by their long interval between initial infection and the appearance of serious symptoms. There are other types of lentiviruses that infect primates other than humans; for example Simian Immunodeficiency Virus (SIV) infects monkeys as well as other primates. We also know that Feline Immunodeficiency Virus (FIV) infects cats. Viruses like SIV and FIV along with their animal host serve as models of the HIV virus to scientist for research.
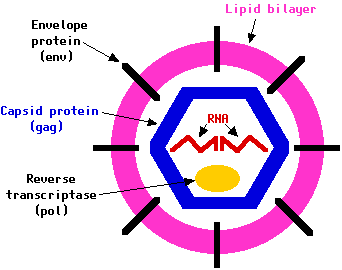
"The HIV virus has a spherical shape with a diameter of 100-120 nm (nanometers)."4 The size of the virus makes it very difficult to view it with a light microscope (which has a maximum magnification of 1,000x), but the virus can be observed with an electron microscope (which has the capacity to magnify objects with greater magnifications).
HIV can only replicate inside cells, but this is not peculiar to HIV because it applies to all other viruses. HIV is composed of an outer coat, which is composed of two layers of fat-like molecules called lipids. This outer coat is called a membrane and is derived from membranes of human cells. Embedded in these membranes are numerous cellular proteins, which look like mushroom-shaped proteins that protrude from the surface. These mushroom-shaped proteins consist of caps and stems. Each cap consists of four HIV molecules called glycoprotein 120 (gp120), and each stem consists of four glycoprotein 41 (gp41) molecules embedded in the membrane of the virus. These proteins are used to recognize CD4 receptors of T cells, attach to them, and infect T cells.
The HIV virus contains a core within the membrane, which is made of an HIV protein called p24 and is surrounded by two copies of viral RNA. There are nine genes within each copy of viral RNA; these genes are gag, pol, env, tat, rev, nef, vif, vpr, and vpu. Each of these genes has a different function during the life cycle of HIV, for example, gag, pol and env contain information needed to make structural proteins. A specific example is the env gene; it codes for gp160, which is the envelope precursor protein that is later broken down to gp120 and gp41. The tat gene enhances viral replication, and vif, vpr, and vpu contain information necessary for the production of proteins, which control the virus ability to infect cells, cause disease, and produce new copies of itself.
HIV Life Cycle
This image was provided by Dr. John
W. Kimball
http://www.ultranet.com/~jkimball/BiologyPages/
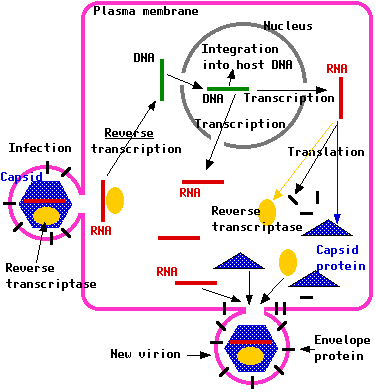
The virus enters the T cell when it encounters a CD4 receptor, which is located on the surface of the T cell. The gp120 of HIV binds tightly to the CD4 receptor and with the help of gp41 the virus and the cell fuse. The virus releases it RNA, proteins, and enzymes into the cell. One of the enzymes that are released into the cell is reverse transcriptase, which is an enzyme that is not produced by human cells. This is an important enzyme for HIV because viral RNA will not be integrated into the nucleus of T cells unless it is converted into viral DNA by reverse transcriptase.
The viral DNA migrates into the cellís nucleus (where host genetic material is stored) and is integrated with the hostís DNA, with the help of an enzyme called viral integrase. HIV DNA, also called the provirus, is duplicated every time the cell divides, and this is how the genetic material of the virus is multiplied.
Going from RNA to DNA is called reverse transcription, where transcription proceeds in a DNA to RNA sequence. Transcription is another important process because HIV is a retrovirus, which means itís an RNA virus. In order to make new viruses the viral DNA must go back to viral RNA using an enzyme called polymerase II as well as other cellular enzymes.
Translation is a process, which converts messenger RNA (mRNA) to proteins and enzymes. Each mRNA molecule is a long single strand of RNA that carries the information of the genetic code of DNA and passes it from the nucleus to the cytoplasm (the cellular material outside the nucleus). Inside the T cell, HIV mRNA is processed in the cellís nucleus and transported to the cytoplasm. HIV mRNA undergoes translation in order to process HIV structural proteins and enzymes that are needed for the assembly of new viruses.
Proteins produced from translation are called immature viral proteins, because they unable to build an infectious virus unless they undergo another process involving an enzyme called protease. In this process, viral proteins are cleaved into shorter chains of proteins that assemble infectious viruses. The different components of the virus are then gathered near the membrane and are pinched off from the cell. The new infectious viruses are then capable of infecting other cells. HIV can also be spread from cell to cell through the fusion of an infected cell with an uninfected cell.
Nucleoside Analogs:
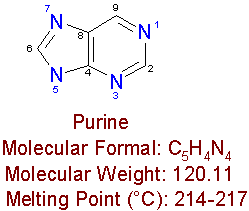
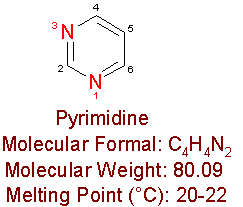
Nucleosides are compounds, which consist of a nitrogen containing base (like purine or pyrimidine) linked to a sugar. Examples of nucleosides are the sub-units of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), which are adenosine, guanosine, cytidine, thymidine, and uracil. Analogs are drugs, which have minor differences in their molecular structures from their parent compounds.
Nucleoside reverse transcriptase inhibitors were the first class of drugs approved for the treatment of AIDS. Reverse transcriptase, as I mentioned earlier, is an enzyme, which catalyzes the synthesis of DNA from RNA. The body breaks down nucleoside analogs into chemicals that stop HIV from infecting uninfected cells in the body, but it does not help cells that have already been infected with the virus.
AZT
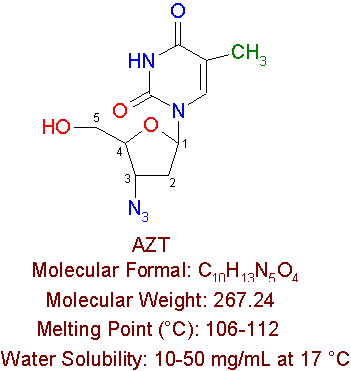
AZT is the first nucleoside reverse transcriptase inhibitor to be approved for treatment of AIDS in the United States. AZT is manufactured by Glaxo Wellcome Pharmaceuticals by the brand name RetrovirÒ , but is also known as azidothymidine, zidovudine, and ZDV. AZT is a thymidine nucleoside analogue in which an azido (-N3) group replaces the 3'-hydroxy (-OH) group.
For treatment, AZT is given in many forms, either in capsules, tablets, syrup, or injection. Syrup is given to newborn infants within 12 hours of birth through 6 weeks of age. The dose is based on body weight, and is usually about 2mg per kilogram or 0.9mg per pound of body weight that is given every six hours. Injection dosage in also according to body weight, and is about 1 to 2 mg per kilogram or 0.45 to 0.9 mg per pound, which is injected slowly into a vein every 4 hours around the clock. Tablets and capsules come in 300mg and 100mg, respectively. They are prescribed at 300mg (1 tablet) twice a day, every 12 hours or 200mg (2 capsules) three times a day, every 8 hours. Capsules are usually used for dose reduction due to side effects.
Patients infected with HIV who have advanced symptoms, early symptoms, or no symptoms at all use AZT to slow the progression of the virus. AZT is not a cure; however, it helps keep HIV from reproducing and slows down the destruction of the immune system. Studies on rats and rabbits showed AZT to have the ability to cross the placenta. In this study, AZT was given by mouth in doses many times larger than the human dose, and caused no birth defects. Instead, AZT decreased the chance of passing HIV to babies during pregnancy and at birth. "A study has also shown that pregnant women treated with AZT were only one-third as likely to pass HIV on to their babies as women who didn't take AZT. This has led to a recommendation that HIV-positive pregnant women be counseled about the potential benefits of AZT in preventing the transmission of HIV to their child."5
Studies show that the best way to administer anti-HIV drugs is in three-drug combinations, better known as triple combination therapy. A combination of AZT, 3TC and indinavir greatly reduces the viral load to undetectable levels and delays the progression of AIDS. Triple combination therapy has been successful because certain anti-HIV drugs attack certain systems in the body better than others do, so a combination of them would reinforce their strength in order to suppress the virus. For example, HIV infects cells in the blood stream, the lymph system, and the central nervous system, but there are only few anti-HIV drugs that cross the blood-brain barrier. AZT is better in attacking the virus in the central nervous system than most other drugs; therefore it would be best to have all HIV patients on AZT.
Common side effects of AZT include loss of muscle, muscle aches, anemia, white blood cell depression, diarrhea, headache, and loss of appetite. Other side effects can also be skin rash, itching, dizziness, nausea, stomach pain, fever, sweating, sore throat, and bone marrow problems.
ddI
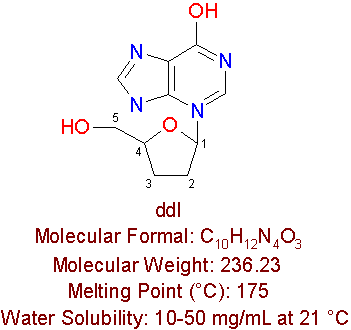
Dideoxyinosine is another approved nucleoside reverse transcriptase inhibitor, which is used for AIDS treatment. Dideoxyinosine is manufactured by Bristol-Myers Squibb Pharmaceuticals by the brand name VidexÒ , but is also known as didanosine and ddI. This drug is a purine nucleoside analogue, which has no substitutions at the 3' position of b -D-Ribofuranose, but has a hydroxy substitution at carbon 6 position of purine. DdI has the same structure as guanosine for the exception of not containing the amino group at the carbon 2 of purine, and not containing a hydroxy group at positions 2' and 3' of the sugar.
DdI comes in three forms, tablets, liquid, or a powder that must be mixed with water or an antacid before using. It must be taken on an empty stomach, which means 2 hours before or 2 hours after eating, as food may decrease the absorption of ddI in the stomach. This drug should not be taken with any acidic beverages, because it contains a special buffer, which keeps ddI from being destroyed in the stomach. Each tablet of ddI contains antacid to prevent stomach acid from destroying the drug. The dosage for ddI depends on body weight and can be changed in case of weight loss or gain. A patient who weighs less than 60 kilograms or 132 pounds, takes 167mg of oral dosage in a form of solution every 12 hours. Patients, who weigh more than 60 kilograms, take 250mg or more. Children, who are taking the pediatric suspension, range from 31 to 125mg every 8 to 12 hours, depending on body weight. Adults, who weigh less than 60 kilogram, take 125mg of tablets every 12 hours, and those who weigh more than 60 kilogram take 200mg every 12 hours. Children, on the other hand, take between 25 to 100mg of tablets every 8 to 12 hours, depending on body weight.
Animal studies have not shown ddI to express an ability to cross the placenta and thus not cause birth defects or other problems, but studies on pregnant women have not been done. So it is unknown whether ddI reduces the chance of an HIV-infected mother infecting her newborn baby.
Side effects of ddI range from more common to rare. Some of the most common side effects are anxiety, diarrhea, headache, irritability, restlessness, dryness of mouth, and difficulty sleeping. The less common side effects of ddI are nausea and vomiting, stomach pain, tingling, burning, numbness, and pain in the hands or feet. Rare side effects are convulsions, which are better known as seizures. Fever and chills, shortness of breath, skin rash and itching, sort throat, swelling of feet or lower legs, unusual bleeding and bruising, unusual tiredness and weakness, yellow skin and eyes are also rare side effects of ddI. The more serious side effects, which are caused by ddI are pancreatitis (inflammation of the pancreas) and peripheral neuropathy. Symptoms of pancreatitis include stomach pain, vomiting, and nausea. The symptoms of peripheral neuropathy include numbness, tingling, burning, and pain in the hands or feet.
ddC
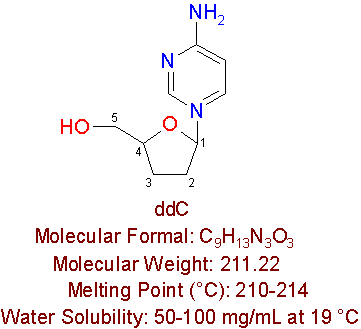
Dideoxyctidine is an approved reverse transcriptase inhibitor, which is also known as ddC, and zalcitabine. Ddc is manufactured by Hoffman-LaRoche Pharmaceuticals by the brand name HIVIDÒ , and is used by HIV-infected people who are intolerant to AZT. This drug is a cytidine nucleoside anlaogue, which differ by not containing a hydroxy group at positions 2' and 3' of cytidine.
Dideoxycitidine comes as 0.375mg and 0.75mg tablets, and are prescribed at 0.75mg together with 200mg of AZT every eight hours or at 0.75mg alone every eight hours. The 0.375mg tablets are used in order to decrease the strength of the drug due to side effects.
The side effects of this drug are similar to ddI; ddc causes serious side effects, which include pancreatitis and peripheral neuropathy. The differences between them are which are most common and which are least common. For example, the most common side effects of ddC are numbness, tingling, burning, and pain in the hand, feet, legs, or arms, but these side effects are the least common of ddI. The less common side effects of ddC are fever, muscle pain, joint pain, skin rash, and ulcers in the mouth and throat.
d4T
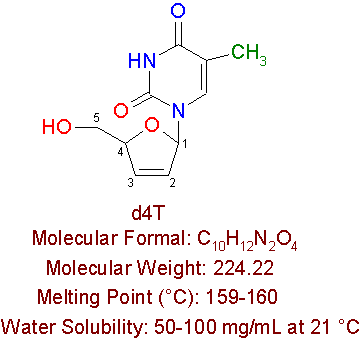
Stavudine, also known as d4T, is another approved reverse transcriptase inhibitor, which is manufactured by Bristol Myers-Squibb Pharmaceuticals by the brand name ZeritÒ . Patients who can not tolerate AZT use this drug. D4T is a dideoxynucleoside pyrimidine analogue, which has the chemical name 2' 3'-didehydro-3'-deoxythymidine.
"D4T comes as 40mg (dark orange), 30mg (light and dark orange), 20mg (light brown), and 15mg (light yellow/dark red) capsules. The starting dose is either 40mg or 30mg (one capsule) twice daily, every twelve hours, depending on weight. The 20mg and 15mg strengths are used for dose adjustments due to side effects. Although D4T is not affected by food, it is best to take it on an empty stomach."6 The dosage of d4T is according to human weight; adults and teenagers who weight 60 kilogram (132 pounds) or more take 40mg two times a day. Those who weight 60 kilogram or less take 30mg two times a day. Studies in animals showed that d4T may cause birth defects when given in very high doses.
Pancreatitis and peripheral neuropathy seem to be serious side effects among most of the nucleoside reverse transcriptase inhibitor. The most common side effects of d4T are numbness, tingling, burning, and pain in the hands or feet. The least common ones are skin rash, joint pain, fever, diarrhea, headache, and muscle pain. Rare side effects of d4T are severe stomach pain, unusual tiredness or weakness, nausea, and vomiting.
3TC
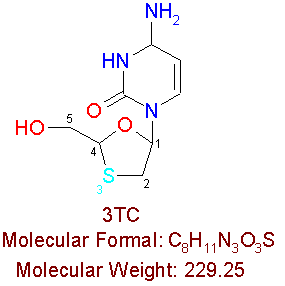
3TC, also known as Lamivudine, is a FDA approved drug, which is used in combination with AZT. EpivirÒ is the brand name of 3TC. This compound is composed of the (-) enantiomer (mirror image) of the racemic mixture 2'-deoxy-3'-thiacytidine, which makes up itís chemical name. The chemical formal of 3TC is almost the same as ddC, except that 3TC contains a sulfur atom at the 3' position.
"3TC comes as a 150mg tablet. It is prescribed at 150mg (1 tablet) twice daily, every 12 hours. Although 3TC is not affected by food, it is best to take it on an empty stomach."7 The common side effects of 3TC include trouble sleeping, fatigue, headaches, diarrhea, decrease in appetite, and nausea.
Protease Inhibitors
Protease Inhibitors are drugs that slow down the spread of HIV inside the body, but they are not considered a cure for HIV infection. "The researchers studying these drugs still have a number of questions about how well they will work and how they should be used."8 Studies of protease inhibitors showed that these drugs are the most powerful anti-HIV drugs that have been used to date clinically. "Certain protease inhibitors can reduce the amount of virus in a person infected with HIV by as much as 99%."9
Protease inhibitors are drugs that prevent HIV protease enzyme from cutting long chains of proteins and enzymes into shorter chains, which are required for production of new infectious copies of the virus. HIV protease inhibitors slow down the spread of the virus by reducing the number of infectious copies of HIV inside the cell.
Protease inhibitors do not completely inhibit the production of new HIV copies in the cell. There are still new copies that are pushed through the walls of infected cells in the presence of HIV protease inhibitors, even though the long chains are not cut up into smaller chains. These viruses are defective so they are unable to infect other cells.
HIV protease
This image was provided by Dr. Martin Markowitz
of Aaron Diamond AIDS Research Center
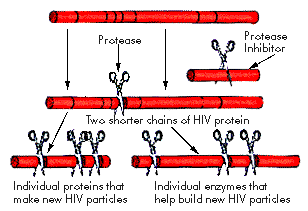
HIV Protease is an enzyme, which cleaves peptide bonds that link amino acid in protein molecules. The HIV virus depends on several enzymes, which it brings or makes inside the cell in order to produce new viruses. The HIV protease is one of these enzymes and its job is to cut long peptide chains into shorter peptide chains. "The Ďscissorsí HIV uses to cut these chains is one of its enzymes, called protease."10 The reason HIV protease is called a Ďchemical scissorsí is because it cuts long chain of proteins into shorter chains. This is a process, which is necessary for the continuation of HIV infection. The stage at which the protease cuts the long chains is towards the end of the HIV life cycle. At this point the virus has already entered the cell and undergone reverse transcription, which converts RNA to DNA.
Difference between Protease Inhibitors and other available anti-HIV drugs
The other available anti-HIV drugs are known as reverse transcriptase inhibitors, and some of them are AZT, ddC, ddI, 3TC, and d4T. The difference between reverse transcriptase inhibitors and protease inhibitors are their targets. "Reverse transcriptase is an enzyme, found mainly in retroviruses, that catalyzes the synthesis of DNA from RNA. It enables the viral RNA to be integrated into the host DNA."11 Reverse transcriptase inhibitors inhibits the HIV replication cycle at an earlier than protease inhibitors.
Since reverse transcriptase inhibitors and protease inhibitors inhibit at different stages in the replication process of HIV, it is believed it would be best if both inhibitors are combined together. Another reason is that drug experts do not expect a bad interaction between them because the liver processes protease inhibitors while reverse transcriptase inhibitors are processed inside non-liver cells and are eliminated from the body through the kidneys. Since these drugs are processed in two separate compartments inside the body, there is less chance of them interacting with one another and cause a harmful side effect. There are other drugs that are used by people who take protease inhibitors, which also get processed in the liver. In these situations the chances of an unfavorable interaction between these drugs and protease inhibitors are much greater.
Getting rid of HIV by Protease Inhibitors
Scientists suspect no one drug can remove the entire virus from an HIV infected person, even though protease inhibitors have shown great success in reducing the viral load in the blood and raised the CD4 T-cell count. HIV does not only infect T-cells, but infects other cells in the body. Some of these infected cells stay dormant, which means they are not active. Dormant cells are infected cells that are waiting to synthesis new viruses.
Protease inhibitors do not get rid of all HIV in the body but they do slow the pace of HIV replication in uninfected cells. Since fewer CD4 cells will be infected and destroyed, a person infected with HIV can stay healthier for a longer period of time by using these drugs than without using them.
Protease Inhibitors that are approved by FDA
Saquinavir
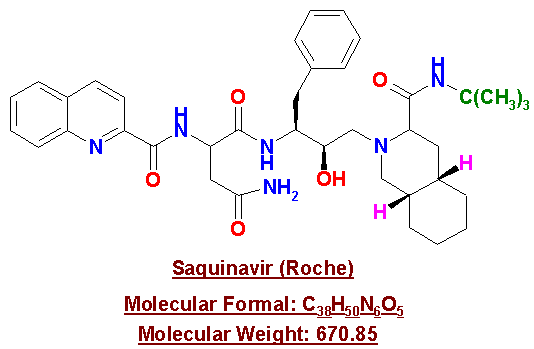
Saquinavir comes as 200mg light brown/green capsules that should be stored at room temperature in a dry place. The recommended dosage of Saquinavir is three 200mg capsules three times daily taken within 2 hours after a full meal. "If taken on an empty stomach, the amount of drug that gets into the blood stream is not enough to work against the virus."12
Research was conducted on Saquinavir in combination with reverse transcriptase inhibitors. The first combination was Saquinavir and AZT. HIV patients were administered with a combination of 1800mg of Saquinavir and 600mg of AZT per day. The patients showed an increase in CD4 count and a decrease of viral RNA. "At study entry, patients, on average, had CD4 counts of about 190 cells/mm3 and viral RNA of about 5.2 logs or 158,000 copies/mL."13 After two weeks of taking the combination of Saquinavir/AZT, the patientís viral RNA load was 1.6 logs, which is about 40 copies/mL, and the CD4 count increased by 70 cells/mm3 after 16 weeks.
The second combination was Saquinavir and ddC. Patients entering this experiment had a baseline CD4 counts of about 160 cells/mm3 and viral RNA levels of about 5.3 logs or 200,000 copies/mL. These patients showed an increase in CD4 count of 30 cells/mm3 and a decline in viral RNA of 0.6 logs or about 4 copies/mL after 48 weeks.
The third combination was of Saquinavir, AZT, and ddC. This combination showed a significantly better reduction in viral RNA load and a higher increase in CD4 counts than in either of the other binary combinations. After 8 weeks, patients showed a viral RNA decline to 0.8 log or 6 copies/mL and an increase of CD4 counts of 35 cells/mm3. "However, both viral load and CD4 responses returned toward pre-treatment levels at 48 weeks. Overall, Saquinavir in combination with AZT and/or ddC provided significantly better antiviral activity compared to similar treatment arms without Saquinavir."14
Many drugs have side effects, which range from mild to severe. Some patients experience these side effects while others do not. Some side effects are present and not felt by the patient, because they are biochemical reactions that occur inside the body without causing pain to the patient. The only way of testing for these biochemical side effects is through laboratory assays. "The most common side effect of Saquinavir is diarrhea, bloating and gas. Other less common side effects include lightheadedness, fatigue, headache, muscle aches, nausea, and vomiting."15
Some drugs should not taken
with other drugs because of harmful interaction they may cause to the patients.
Saquinavir is no different; it should not be taken with certain drugs.
Indinavir
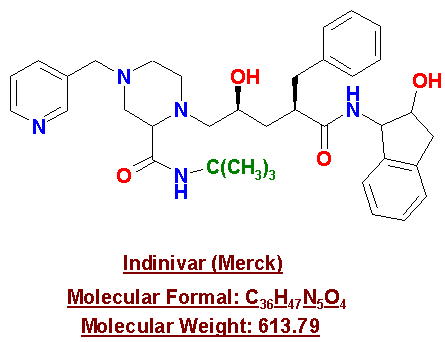
Indinavir, also known as CrixivanTM, is an antiretroviral medication that is manufactured by Merck Pharmaceuticals. Indinavir works in the same manner as Saquinavir; it interferes with the life cycle of HIV by inhibiting the protease enzyme and does not allow the virus to replicate inside the cell.
Indinavir comes in a 200mg blue capsule or a 400mg green tablet that should be stored at room temperature. The recommended dosage of Indinavir is 800mg every 8 hours. This dosage can be administered by 2 tablets or 4 capsules three times a day. "It must be taken every eight (8) hours on an empty stomach, and with plenty of water (6 to 8 full glasses of water daily). If taken with food, the amount of drug that gets into the blood stream is not enough to work against the virus."17
Research experiments were performed on Indinavir to determine which combination of drugs would be most powerful to fight HIV. These experiments were conducted on HIV-infected people who had received no more than 2 weeks of prior AZT therapy. These patients had a CD4 counts of less than 500 cells/mm3 and a viral RNA load of greater than 20,000 copies/mL. They were placed into three categories, Indinavir monotherapy, AZT monotherapy, and combination of AZT and Indinavir. Indinavir monotherapy were administered with 600mg capsule every 6 hours, AZT monotherapy were administered with 200mg three times a day, and combination therapy where they received both dosages. Each category consisted of 20 to 25 patients and the study duration was 24 weeks.
A reduction in viral RNA load was observed in all participants at week 24. A reduction of 0.5 log (about 3 copies/mL) was observed for those patients who participated in the AZT monotherapy. There was a reduction of 1.5 log (about 31 copies/mL) for those who received the Indinavir monotherapy. The patients who received the combination of Indinavir and AZT had a reduction of 2.5 log (about 316 copies/mL). These results showed that the best way to administer Indinavir is with another drug like AZT.
In the same study, a few of the patients that received the combination of Indinavir and AZT had a decline of only 1 log (about 10 copies/mL) in their viral RNA load. "Dr. Emini explained that the diminished level of viral suppression was due, in part, to the selection of Indinavir-resistant HIV strains."18 In contrast, a few of the patients had a decrease in viral RNA load by as much as 4 log (about 10,000 copies/mL) at week 24.
A virus can adapt to different environments in much the same way as humans. This creates a problem when fighting a virus like HIV, because it can become resistant to drug like AZT, Saquinavir and Indinavir. In order to monitor this problem further studies had taken place with patients; a blood sample was taken for laboratory analyses to determine the genetic changes that have taken place in the virus. "At week 24, AZT-resistant mutant strains were detected in 11 out of 16 patients from the AZT mono-therapy group, 1 out of 13 patients from the Indinavir monotherapy group, and 1 out of 13 patients from the Indinavir plus AZT group. Taken together, these findings suggested that use of Indinavir in combination with AZT may delay the emergence of AZT-resistant mutant strains over the stud duration (24 weeks)."19
Indinavir has side effects, which are the same as Saquinavir. The most common side effects of Indinavir are headaches, diarrhea, rash, nausea, and loss of appetite. "Although Indinavir should be taken on an empty stomach, the nausea can be controlled by taking a dose with a light snack such as a bowl of corn flakes with skim milk or a couple pieces of toast with jelly but no butter."20 Another side effect of Indinavir is the formation of kidney stones, which could be due to the crystallization of Indinavir. Kidney stones are usually easy to detect because of the painful urination or the pain felt around the groin area. One of the ways of preventing this problem is by drinking enough water and other liquids during the day, which is why drinking 6-8 glasses of water a day is recommended while using Indinavir.
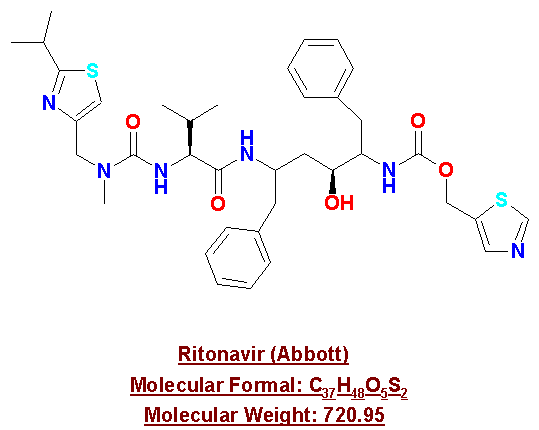
Ritonavir