
Workshop CHE 166
Problem Set #1
1. Classify the following processes as either a physical or a chemical property:
a) ice melts at 0 oC
b) newspaper burns
c) glass breaks
d) the vitamin content of foods in contact with air decreases
2. Classify each of the following as an element, a compound, or a mixture:a) air
b) table salt
c) baking soda
d) wine
e) oxygen gas
3. What is the SI unit and symbol for energy? What is the SI unit and symbol for one one-thousandth of this unit?4. How many milliliters is 0.005 L?
5. The diameter of an atom is approximately 1 x 10-8 cm. How many nanometers is this?
6. Given that 1 in. = 2.54 cm (exactly), determine how many inches are in 1 meter.
7. Express 450 kg in megagrams.
8. Determine the number of
a) square meters in 1 square kilometer
b) cubic decimeters in 1 liter.
9. How many cubic centimeters are there in exactly one cubic meter?
10. Convert 3.0 x 1010 cm/s to units of
a) km/s
b) km/hr
c) mm/hr
11. How many significant figures does the number 0.00721 contain? Write this number using scientific notation.12. The number 0.009604870 x 105 contains how many significant figures? Write this number using scientific notation.
13. Calculate the value of k for the following expression rounded to the correct number of significant figures.
k = (1.5 x 10-4 x 61.3) + 2.01
14. How many significant figures does the sum of the following contain?
8.5201 + 1.93
15. How many significant figures does the difference (218.7201 - 218.63) contain?
16. Calculate the value of t. How many significant figures are appropriate to show in the result? How many decimal places are in the correct result?
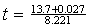
17. Calculate the value of the following math operation. How many significant figures are appropriate to show in the result? How many decimal places are in this result?
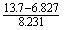
18. An irregularly shaped piece of metal with a mass of 125 g is placed into a graduated cylinder that contains 25.00 mL of water. This raises the water level to 56.00 mL. What is the density of the metal?
19. Given the following densities at 25 oC:
magnesium: 1.7 g/cm3
graphite: 1.8 g/cm3
iron: 7.9 g/cm3A block of iron has a mass of 826 g. What is the mass of a block of magnesium that has the same volume as the block of iron?
20. Bromine is a red liquid at 25 oC and has a density of 3.12 g/cm3. What is the volume of 28.1 g of liquid bromine?
21. If a car has an EPA mileage rating of 30 miles per gallon, what is this rating in km/L?
22. If the price of gasoline is $1.14 per U.S. gallon, what is the cost per liter?
23. The highest temperature recorded in Phoenix, Arizona was 122 oF. Express this temperature on the Celsius scale. Express this temperature on the Absolute scale.
24. Ammonia boils at -33.4 oC. What is this temperature on the Fahrenheit scale? What is this temperature on the Absolute scale?
25. When 7.02 oC is converted to the Fahrenheit scale, how many significant figures are there in the result? When the same Celsius temperature is converted to the Absolute scale, how many significant figures are there in this result?
26. The recommended daily allowance (RDA) of calcium is 1.2 g. Calcium carbonate contains 12.0% calcium by mass. How many grams of calcium carbonate are needed to provide the RDA of calcium?
27. Americans combined drive about 4.0 x 109 miles per day and get an average of 20 miles per gallon. For each kilogram of gasoline that is burned about 3 kg of carbon dioxide are produced. How many kilograms of CO2 are emitted by US cars into the atmosphere each day? One gallon of gas weighs about 3.5 kg.